The LYSARC conducts various clinical studies in the field of lymphoma research, in collaboration with the LYSA clinical research network.
The LYSARC has contributed to important scientific production on lymphomas by the LYSA clinical research network.
Accelerating lymphoma research
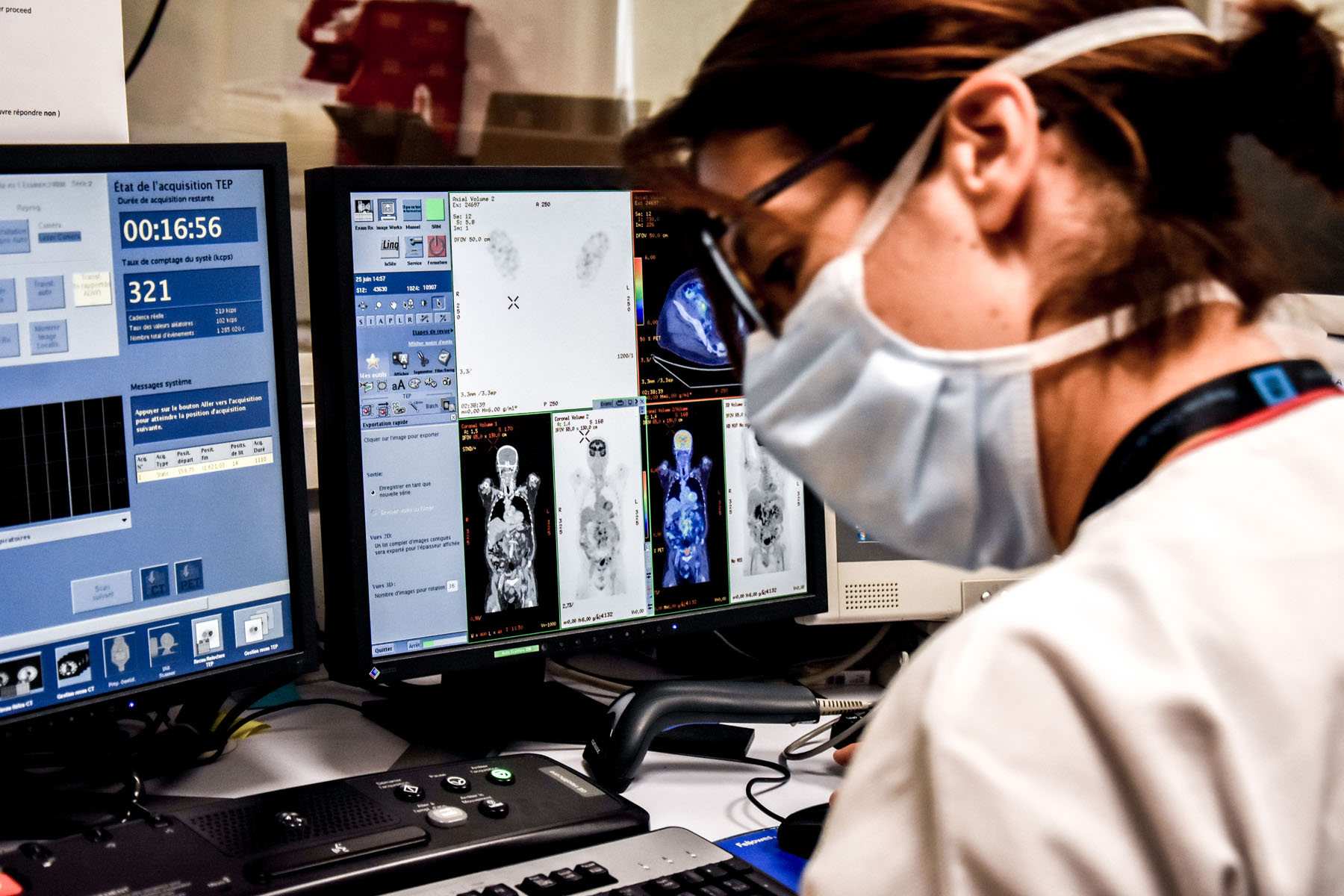
The LYSARC, in collaboration with the LYSA independent clinical research network, conducts major clinical studies in the field of lymphoma research. The AHL 2011 phase 3 study and the real-life study REALYSA are two examples of this. The results of the AHL 2011 study defined a new care standard for advanced Hodgkin lymphomas: the PET-guided treatment strategy. As for the REALYSA, this is an unprecedented real-life study with 6,000 French patients suffering from various types of lymphomas, monitored over a period of 9 years. Objectives: improving the available knowledge and opening the possibility of new lymphoma research projects.
News


Ensemble contre le lymphome
An independent network of clinical lymphoma research professionals, acknowledged as a Cooperating Group
An operational lymphoma research structure that conducts the LYSA projects